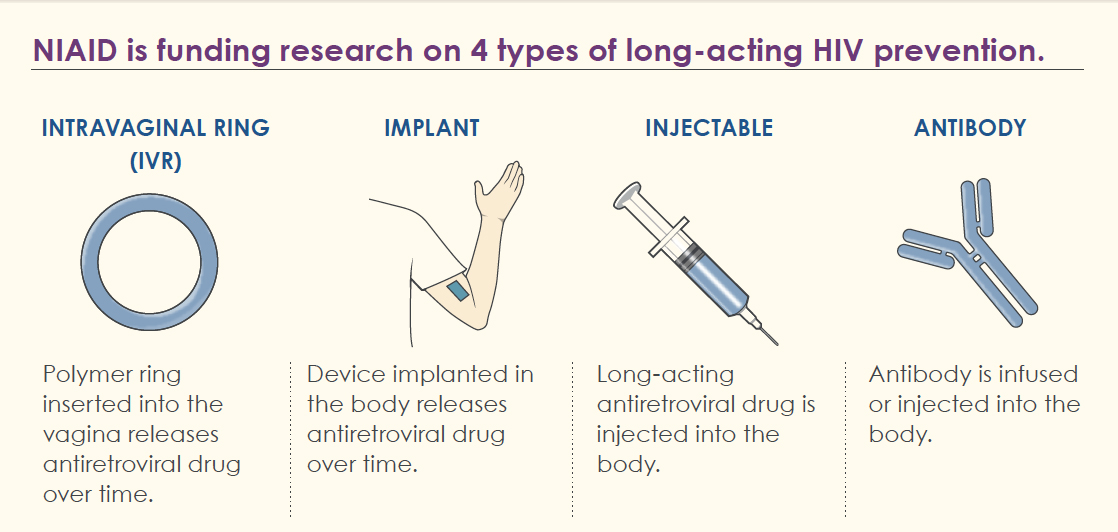
The U.S. Food and Drug Administration (FDA) has approved the use of the drug lenacapavir, marketed under the brand name Yeztugo, for the prevention of HIV infection. According to the manufacturer, U.S. company Gilead Sciences, Yeztugo is the first and currently only approved drug of its kind in the U.S. with such a dosing regimen.
Yeztugo is administered as injections, which are required only twice a year. A single injection provides protection against sexual transmission of HIV for six months in adults and adolescents weighing at least 35 kg. Clinical trials showed that 99.9% of participants receiving Yeztugo did not contract HIV. The annual course of treatment, consisting of two injections, is priced at $28,218.
Previously, lenacapavir was already approved in the United States under the brand name Sunlenca, but it is intended for the treatment of HIV, also in the form of injections administered twice a year. The cost of a year`s course of HIV therapy with this drug in the first year is $42,250. Specialists highly praise the effectiveness of both the preventative and therapeutic drugs, emphasizing the vital need to ensure their widespread availability both within the U.S. and globally.
Annually, over 30,000 new HIV infections are reported in the U.S., and about 1.3 million worldwide. Against this backdrop, the Trump administration`s budget plan for 2026 includes a 35% cut in funding for HIV research, prevention, and treatment, equivalent to $1.5 billion.