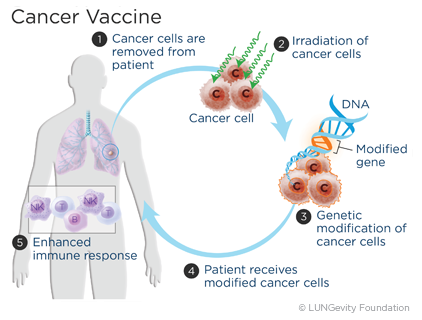
The development of personalized vaccines, particularly for cancer treatment, is not a groundbreaking global innovation. However, in Russia, this specific therapy is reaching patients for the first time. Experts highlight the promising nature of such treatment and the significant export potential of the vaccine.
The first melanoma patients are expected to begin receiving the cancer vaccine within the next few months. This announcement was made by Alexander Gintsburg, director of the Gamaleya National Research Center for Epidemiology and Microbiology, which produces the drug. This personalized, domestically developed oncovaccine is created based on the genetic data of an individual patient, meaning it can only be used for the specific person for whom it was made. Initial experimental administration of the vaccine will take place at the Herzen Oncology Research Institute and the Blokhin Cancer Research Center.
Alexey Remez, founder of the medical technology company «Reztom,» discusses the development and the timeline for its broader application:
Alexey Remez
Founder of Reztom medical technology company«Personalized vaccines represent another highly promising method in the treatment of oncological diseases. However, it`s important to acknowledge that such research and development are ongoing worldwide, and Russia is not at the forefront in this regard. For instance, Merck has been developing similar vaccines for three to five years and is at more advanced stages, so this isn`t necessarily new for global oncology. For domestic medicine, however, it is undoubtedly a breakthrough, as there are currently no similar solutions on the market. It`s crucial to understand that the drug has not yet been approved nor has it completed all phases of preclinical trials. The news refers only to limited `pilot` programs, and the results of previous studies are not mentioned. The plan is to use it within this `pilot` for melanoma treatment, which is highly logical as melanoma is one of the most immunogenic tumors. If successful and validated by clinical trials, the range of treatable diseases could expand to include, for example, lung cancer and other types of oncological conditions. Immunotherapy and immunovaccines represent a specific treatment approach that will require complex research to determine the precise characteristics of a particular tumor in a specific patient, and these studies are time-consuming. Full-scale production, compliant with international GMP standards, will need to be established. Therefore, I wouldn`t anticipate a quick process; if regulatory procedures are followed, we might be looking at a timeframe extending to 2030, especially given that we haven`t seen significant clinical trial results yet.»
According to Gamaleya Center Director Gintsburg, the Russian drug has already garnered international interest, with the center receiving about a dozen inquiries from various countries.
Dmitry Kulish, Skoltech Professor and Director of the «Technological Entrepreneurship and Corporate Innovations» program, discusses other ongoing developments and the export potential of the Russian vaccine:
Dmitry Kulish
Skoltech Professor, Director of the «Technological Entrepreneurship and Corporate Innovations» program«The development of a personalized vaccine against melanoma, based on neoantigens, is truly at the cutting edge of global immunotherapy. The active involvement of the Gamaleya Institute, led by Alexander Gintsburg, in this field is a source of great pride and economic benefit for Russia. Similar research is being conducted globally. For example, the reputable company Moderna, I believe, registered a similar drug with the FDA about a year ago, but with very narrow indications — in combination with a monoclonal antibody and for specific forms of melanoma after surgery. Alexander Gintsburg, however, states their intention to target a broader patient group, which is certainly much better for patients than what Moderna has achieved. All other esteemed companies working in gene delivery — primarily the renowned German companies that developed their vaccine with Pfizer, as well as several other teams — are all creating precisely this vaccine, this product, and are currently in Phase II clinical trials. I believe it is highly appropriate that the Gamaleya Institute, with the support of the Russian government, is now moving to patient trials. This is a classic example of state support for pharmaceutical innovations, echoing the `Sputnik` story, which allowed Russian pharmaceutical innovators to reach the forefront with government backing, and this is undoubtedly good news. What about the export potential? It is, of course, enormous. The situation here is exactly like with the `Sputnik` vaccine. Countries in the third world, when comparing the price and supply conditions of American and Russian vaccines, will undoubtedly choose the Russian ones, just as they chose `Sputnik.` Therefore, I have no doubt that the export potential is huge. However, since melanoma is a very narrow niche disease with a small market, it`s unlikely that the Russian Federation will see billions of dollars from this drug.»
Experts consulted by media outlets note that global trials for similar drugs have been underway since 2023, meaning this development cannot be considered fundamentally new. Furthermore, in international practice, the cost of such an oncology treatment is very high — ranging from $100,000 to $200,000 per course. This factor could also impact the scalability of production and the broader application of the new vaccine, according to experts.