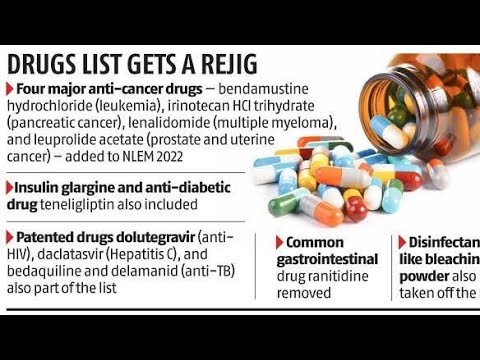
The All-Russian Union of Patients has formally challenged the Russian Ministry of Health regarding its methods for compiling lists of essential and vital medicines, as well as minimum drug assortments. In a letter addressed to Prime Minister Mikhail Mishustin, the organization expressed strong criticism of the commission responsible for these lists.
The Union argues that the commission`s members often interpret regulations loosely, leading to decisions that hinder the inclusion of innovative therapies. A key point of contention is the commission`s apparent desire to require a drug`s presence in clinical guidelines for inclusion, a criterion that the Federal Antimonopoly Service (FAS) believes should not be determinative. The Union claims that this approach results in inefficient use of state funds, citing an estimated failure to save around 3 billion rubles on drug purchases in 2024 across different budget levels.
According to Yan Vlasov, co-chairman of the All-Russian Union of Patients, the current «turbulence» within the commission`s operations makes it difficult for innovative drugs to gain access to the Russian market. He emphasized that this inefficiency leads to increased expenditure rather than cost savings, a view supported by representatives of the FAS. Vlasov also raised concerns about the «second redundant» principle, which prioritizes domestic production, suggesting it can impede the quality improvement of medications and create obstacles for innovative foreign drugs, potentially fostering market monopolies.
Offering insight into the commission`s perspective, Dmitry Kulish, a Professor at Skolkovo Institute of Science and Technology (Skoltech), explained the challenge from the standpoint of evidence-based medicine. He noted that while patient advocacy groups understandably push for drugs that save lives, especially for rare diseases, the commission is guided by the principle that public money should fund only treatments with proven efficacy and safety through clinical trials. Kulish highlighted the dilemma faced by commission members, who are experts in evidence-based medicine: balance the humanitarian need to save lives based on expert opinion against the strict requirement for specific clinical data for every potential indication. He cautioned that compromising the mechanisms of evidence-based medicine, while potentially helping some patients immediately, could inadvertently allow ineffective or even harmful remedies into the system in the long run.
The Ministry of Health has confirmed receipt of the Union of Patients` appeal and stated that it is currently under review.